Research: It turns out the boosters were two Falcon 9 first stages. Falcon 9 was the test vehicle for the SpaceX Reusable Launch System Development Program. This is a program created by SpaceX to develop a rocket capable of launching over and over while also landing itself. This takes time and money to build. The research has been immense. The rocket cost over $90,000,000 USD to build. And it hasn't been entirely successful. The Falcon Heavy's side boosters landed perfectly, The main engine, not so much. The rocket failed to land safely and it was back to the drawing board

Falcon heavy booster landings

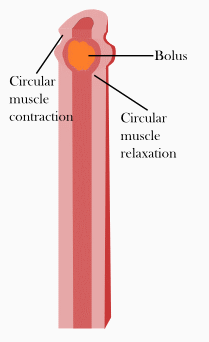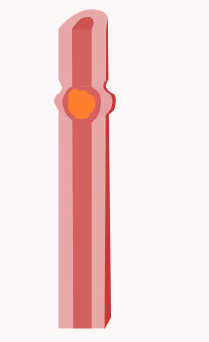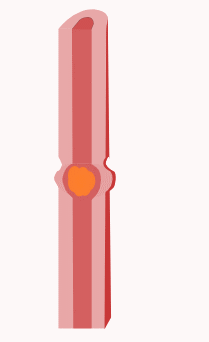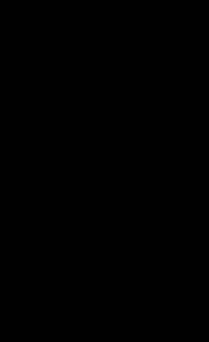









 (2)
(2) (1-2 *step 4*)
(1-2 *step 4*) (2-2 *step 4*)
(2-2 *step 4*) (5)
(5)


